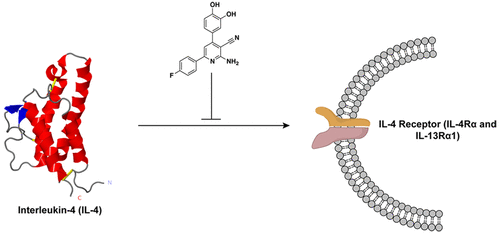
Interleukin-4 (IL-4), a multifunctional cytokine, is an important regulator of inflammation. When deregulated, IL-4 activity is associated with asthma, allergic inflammation, and multiple types of cancer, making it a potentially impactful target for therapies in a variety of illnesses. While antibody-based inhibitors targeting IL-4 have been tested in clinical trials, they have so far not succeeded as therapeutics. Small-molecule inhibitors of IL-4 provide certain advantages over antibody-based inhibitors, but no small-molecule inhibitors to the soluble IL-4 cytokine have yet been reported, due in part to the challenge of inhibiting protein-protein interactions like the interface between the cytokine and its natural receptor.
From a small molecule microarray screen against purified IL-4, we prioritized a nicotinonitrile scaffold, 52, as a direct binder of the cytokine. Compound 52 has 1.8 µM binding affinity for IL-4 (Kd by SPR) and 3.1 µM potency (EC50) in a cellular assay for type II receptor IL-4 signaling in cells (STAT6 phosphorylation levels by western blot and immunofluorescence). This first IL-4 small-molecule inhibitor has implications for numerous immune-related disorders and inform future drug discovery and design efforts for these challenging protein targets.